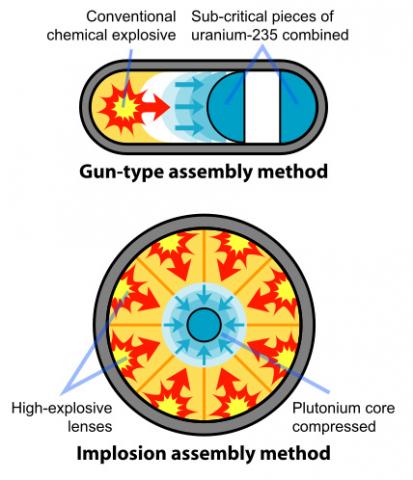
The immense destructive power of atomic weapons derives from a sudden release of energy produced by splitting the nuclei of the fissile elements making up the bombs’ core. The U.S. developed two types of atomic bombs during the Second World War. The first, Little Boy, was a gun-type weapon with a uranium core. Little Boy was dropped on Hiroshima. The second weapon, dropped on Nagasaki, was called Fat Man and was an implosion-type device with a plutonium core.
Fission
The isotopes uranium-235 and plutonium-239 were selected by the atomic scientists because they readily undergo fission. Fission occurs when a neutron strikes the nucleus of either isotope, splitting the nucleus into fragments and releasing a tremendous amount of energy. The fission process becomes self-sustaining as neutrons produced by the splitting of atom strike nearby nuclei and produce more fission. This is known as a chain reaction and is what causes an atomic explosion.
When a uranium-235 atom absorbs a neutron and fissions into two new atoms, it releases three new neutrons and some binding energy. Two neutrons do not continue the reaction because they are lost or absorbed by a uranium-238 atom. However, one neutron does collide with an atom of uranium-235, which then fissions and releases two neutrons and some binding energy. Both of those neutrons collide with uranium-235 atoms, each of which fission and release between one and three neutrons, and so on. This causes a nuclear chain reaction. For more on this topic, see Nuclear Fission.
Criticality
In order to detonate an atomic weapon, you need a critical mass of fissionable material. This means you need enough U-235 or Pu-239 to ensure that neutrons released by fission will strike another nucleus, thus producing a chain reaction. The more fissionable material you have, the greater the odds that such an event will occur. Critical mass is defined as the amount of material at which a neutron produced by a fission process will, on average, create another fission event.
The Difference Between the Bombs
Little Boy and Fat Man utilized different elements and completely separate methods of construction in order to function as nuclear weapons. Little Boy detonated due to a fission chain reaction involving the isotope U-235 of uranium, while Fat Man used plutonium’s Pu-239 form.
Little Boy
Little Boy was powered by the uranium isotope U-235 in a process that didn’t come easily to the many Manhattan Project scientists working on the uranium extraction and enrichment process. Most uranium found naturally in the world exists as uranium-238, leaving only 0.7% of naturally existing uranium as the U-235 isotope. When a neutron bombards U-238, the isotope often captures the neutron to become U-239, failing to fission, and thus failing to instigate a chain reaction that would detonate a bomb. The first challenge of the project was thus to determine the most efficient way to separate and purify uranium-235 from the overly-abundant uranium-238 – standard methods of separation could not be used due to the strong chemical similarity between the two isotopes. In order to avoid wasting time on one new method that could later prove insufficient to produce enough U-235 to allow the atomic bomb to reach critical mass, General Leslie Groves consulted with lead scientists of the project and agreed to investigate simultaneously four separate methods of separating and purifying the uranium-235: gaseous diffusion, centrifuge, electromagnetic separation and liquid thermal diffusion.
Once enough U-235 was obtained to power the bomb, Little Boy was constructed using a gun-type design that fired one amount of U-235 at another to combine the two masses. This combination created a critical mass that set off a fission chain reaction to eventually detonate the bomb. The two masses of U-235 had to combine with one another quickly enough to avoid the spontaneous fission of the atoms, which would cause the bomb to fizzle, and thus fail to explode.
Fat Man
Powered by plutonium, Fat Man could not use the same gun-type design that allowed Little Boy to explode effectively – the form of plutonium collected from the nuclear reactors at Hanford, WA for the bomb would not allow for this strategy. The Hanford plutonium emerged from the reactors less pure than the initial plutonium extracted from Ernest O. Lawrence’s Berkeley Lab, instead containing traces of isotope plutonium-240, as opposed to the desired plutonium-239. Plutonium-240’s higher fission rate would cause the atoms to undergo spontaneous fission before the gun-type design could bring two masses of plutonium together, which would lower the energy involved in the actual detonation of the bomb.
Thus, a new design was required. Physicist Seth Neddermeyer at Los Alamos constructed a design for the plutonium bomb that used conventional explosives around a central plutonium mass to quickly squeeze and consolidate the plutonium, increasing the pressure and density of the substance. An increased density allowed the plutonium to reach its critical mass, firing neutrons and allowing the fission chain reaction to proceed. To detonate the bomb, the explosives were ignited, releasing a shock wave that compressed the inner plutonium and led to its explosion.
| Atomic Glossary |
|---|
| Atom: building blocks of matter; made up of a small, dense nucleus surrounded by a cloud of electrons (negatively-charged particles) |
| Nucleus: makes up the center of the atom; consists of a number of positively-charged protons and a neutral (no charge) neutrons. An atom is classified by the number of protons and neutrons in its nucleus. The number of protons determines which chemical element the atom is (e.g. uranium), and the number of neutrons determines which isotope of that element the atom is (e.g. uranium-235). |
| Isotope: Isotopes of an element possess the same number of protons in their nuclei but have different numbers of neutrons. |
| Fission: the process by which an atom’s nucleus is split into smaller particles; results in the release of neutrons and lots of energy. |
| E=mc2: Equation made famous by Albert Einstein. Explains how a tiny amount of matter contains a tremendous amount of energy. |